On 03/31/2023, the European Commission published in the Official Journal Delegated Regulation (EU) 2023/707, which amends the CLP Regulation, establishing new hazard classes and new criteria for the classification, labeling, and packaging of substances and mixtures. This regulation applies from April 20, 2023, but as always, the following transition periods are provided to give suppliers of substances and mixtures time to comply with the new classification and labeling requirements
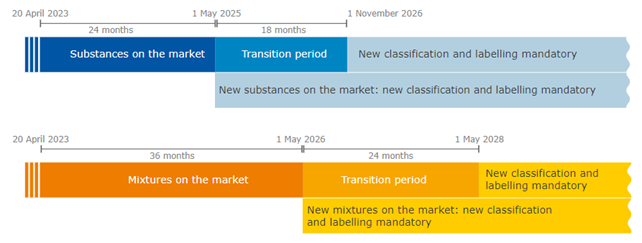
- For new substances on the market, companies will have to comply with the new rules from May 1, 2025, while, for substances already on the EU market, they will have until November 1, 2026.
- For new mixtures, the new CLP hazard classes will apply from May 1, 2026, while for existing mixtures, companies will have until May 1, 2028, to update classification and labeling.
Some tips for fulfilling the new obligations:
– Take note of transition periods for substances and mixtures and ensure that you have fulfilled your duties by these dates at the latest.
– Review the classification and labeling of the substances and mixtures in your portfolio, based on the criteria for the new hazard classes, taking into account all the most up-to-date information available.
– Update REACh registration dossiers with the new information. The new hazard classes for the classification, labeling and packaging of substances and mixtures will be included in the IUCLID software as of April 29, 2024.
– If substance/mixture suppliers are part of a joint registration (joint submission) under REACH, initiate discussion to agree on the classification and labeling of their substances and as appropriate update registration dossiers without undue delay.
– Update the C&L notification to the inventory.
– Evaluate whether an update or a new notification to the poison control center is necessary. Mixtures previously not classified for human health hazards and physical hazards may need to be classified for new hazards.
– Update the safety data sheets (SDSs) with the new classification and labeling and any new information needed to reflect the new hazards. It may be necessary to compile an SDS/provide an SDS upon request for substances or mixtures that were previously unclassified or for which there was no requirement to prepare one. date safety data sheets (SDSs) with the new classification and labeling and any new information needed to reflect the new hazards. It may be necessary to compile an SDS/provide an SDS upon request for substances or mixtures that were previously unclassified or for which there was no requirement to prepare one.
Guides available for adressing news CLP hazards:
In collaboration with EFSA, ECHA is preparing an update of the CLP Criteria Application Guide to include guidance on new hazard classes. The updated guidance is expected to be published in mid-2024. Until now, the following documents can be consulted:
The Draft Version of the CLP Criteria Application Guide: https://echa.europa.eu/it/support/guidance/consultation-procedure/ongoing-clp
- For ED Effects: EFSA/ECHA Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and EC No 1107/2009
https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5311
The list of ED-assessed substances that have been brought to the ECHA ED Panel for discussion contains 119 entries (last update: April 4, 2024) and can be found at:
https://echa.europa.eu/it/ed-assessment
- For PBT/vPvB: Guidance on Information Requirements and Chemical Safety Assessment, Part C: PBT/vPvB assessment (R11), Version 4.0 December 2023 https://echa.europa.eu/documents/10162/17224/information_requirements_r11_en.pdf/a8cce23f-a65a-46d2-ac68-92fee1f9e54f
The list of substances under PBT/vPvB assessment that have been brought to the ECHA PBT Panel for discussion, updated as of April 11, 2024, contains 231 entries and can be found at: https://echa.europa.eu/it/pbt
- For PMT/vPvM: new guidelines are being developed.







